Short Answer Type
Short Answer TypeOn the basis of the following observations made with aqueous solutions, assign primary and secondary valencies to metals in the following compounds:
|
Formula |
Motes of AgCl precipitated with excess AgNO3 |
|
(i) PdCl2.4NH3 |
2 |
|
(ii) NiCl2.6H2O |
2 |
|
(iii) PtCl4.2HCl |
0 |
|
(iv) CoCl3.4NH3 |
1 |
|
(v) PtCl2.2NH3 |
0 |
.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeChelation can be of three major types:
(i) Didentate chelation: When chelation is done by didentate ligand it is called Didentate chelation, e.g., [PtCl2(en)] in this en is a didentate ligands.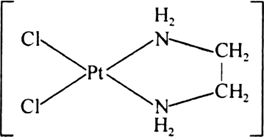
(ii) Terdentate chelation: When chelation is done by a terdentate (tridentate) ligand it is called tredentate chelation e.g., [PtCl(dien)+, dien is a terdentate ligand.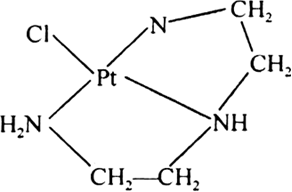
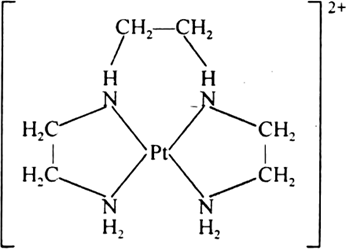
(iii) Tetradentate chelation: When chelation is done by tetradentate ligand it is called tetradentate chelation e.g., [Pt(trien)]2+, trien is a tetradentate ligand.