 Long Answer Type
Long Answer TypeThe degenerate d-orbitals (in a spherical field environment) split into two levels i.e., eg and t2g in the presence of ligands. The splitting of the degenerate levels due to the presence of ligands is called the crystal-field splitting while the energy difference between the two levels (eg and t2g) is called the crystal-field splitting energy. It is denoted by ∆o.
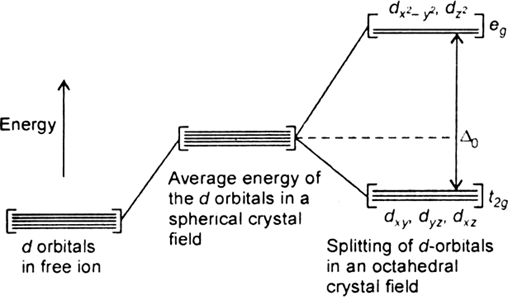
Fig. d-orbital splitting in an octahedral crystal field.
The formation of complex depend on the crystal field splitting, ∆o and pairing energy (P).
i)If ∆o < P, the fourth electron enters one of the eg orbitals giving theconfiguration t2g3. Ligands for which ∆o < P are known as weak field ligands and form high spin complexes.
ii) If ∆o > P, it becomes more energetically favourable for the fourth electron to occupy a t2g orbital with configuration t2g4 eg0. Ligands which produce this effect are known as strong field ligands and form low spin complexes.
 Short Answer Type
Short Answer TypeGive the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
K3[Co(C2O4)3]
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
cis-[Cr(en) 2Cl2]Cl
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
(NH4)2[CoF4]
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
[Mn(H2O)6SO4
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
K[Cr(H2O)2(C2O4)2]. 3H2O
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
CrCl3(py)3
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
Cs[FeCl4].
