 Long Answer Type
Long Answer TypeDiscuss the nature of bonding in metal carbonyls.
Carbonyls are formed by the transitional elements because of the presence of vacant d-orbitals. In carbonyl the oxidation state of the metal is zero.
During the formation of carbonyls the vacant d-orbitals of metal are over-lapped by the filled carbon σ-orbitals. This results the formation of σ-bond between metal and C of CO due to the donation of electron pair of carbon into the vacant d-orbitals of metal. On the other hand a -overlap takes place by a back donation of electrons from a filled d-orbitals of metal into the vacant antibonding p-orbitals of CO and a p M → C bond is formed.![]()
Donation of lone pair of electrons by the C of CO into vacant d-orbital of the metal (sigma overlap)
.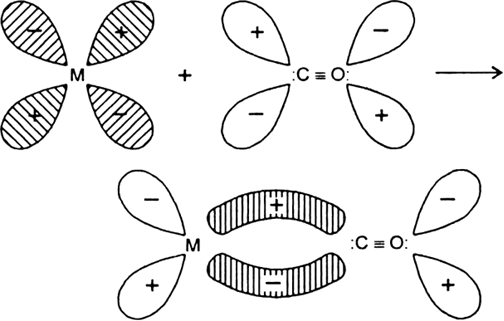
Fig. Donation of electrons from filled d-orbitals of metal into vacant antibonding p - orbital of CO(Pit overlap).
 Short Answer Type
Short Answer TypeGive the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
K3[Co(C2O4)3]
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
cis-[Cr(en) 2Cl2]Cl
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
(NH4)2[CoF4]
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
[Mn(H2O)6SO4
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
K[Cr(H2O)2(C2O4)2]. 3H2O
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
CrCl3(py)3
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
Cs[FeCl4].
