 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeGive the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
K3[Co(C2O4)3]
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
cis-[Cr(en) 2Cl2]Cl
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
(NH4)2[CoF4]
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
[Mn(H2O)6SO4
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
K[Cr(H2O)2(C2O4)2]. 3H2O
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
CrCl3(py)3
CrCl3(py)3
IUPAC name: Trichloridotripyridinechromium(III) oxidation state +3.
Electronic configuration for d3 =t2g3 c
oordination number =6 .
shape : Octahedral.
Sterochemistry: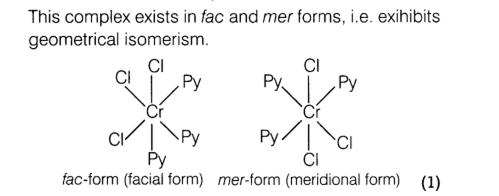
Stereochemistry both isomers are optically active. Therefore, a total of 4 isomers exist.
Magnetic moment
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
Cs[FeCl4].
