 Short Answer Type
Short Answer TypeDeduce the magnetic behaviour of each of the following:
(i) [Cr(NH3 )5Cl]2+
(ii) Fe(CO)5 [At. No. Cr = 24, Fe = 26]
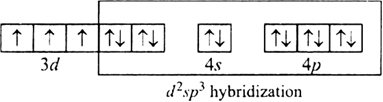
It is paramagnetic due to presence to unpaired electrons.
Since it have three unpaired electron therefore magnetic moment will be.
(ii) Fe(26) has outer electronic configuration 4s2,3d6.
Fe(O) has outer electronic configuration 4s°, 3 d8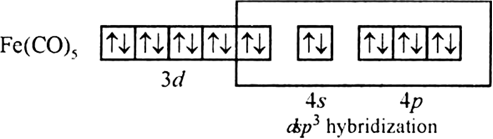
It is diamagnetic because there is no unpaired electron.
Using valence bond theory predict the geometry and magnetic behaviour of [Cr(NH3)6]3+ ion [Cr = 24].
Using valence bond theory, predict the shape and magnetic character of [Ni(CO)4] [Ni = 28].
What type of isomerism is exhibited by the following pair:
[Co(NH3)5Br]SO4 and [Co(NH3)SO4] Br
Give a chemical test to distinguish them.
Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28]
Explain the following:
Cobalt metal complex is pink when it is octahedral [Co(H2O)6]2+ and it is blue when tetrahedral [CoCl4]2–
