 Short Answer Type
Short Answer TypeDeduce the magnetic behaviour of each of the following:
(i) [Cr(NH3 )5Cl]2+
(ii) Fe(CO)5 [At. No. Cr = 24, Fe = 26]
Using valence bond theory predict the geometry and magnetic behaviour of [Cr(NH3)6]3+ ion [Cr = 24].
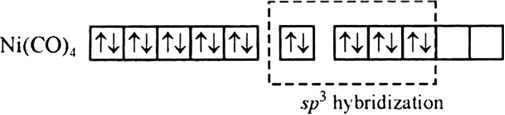
Using valence bond theory, predict the shape and magnetic character of [Ni(CO)4] [Ni = 28].
What type of isomerism is exhibited by the following pair:
[Co(NH3)5Br]SO4 and [Co(NH3)SO4] Br
Give a chemical test to distinguish them.
Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28]
Explain the following:
Cobalt metal complex is pink when it is octahedral [Co(H2O)6]2+ and it is blue when tetrahedral [CoCl4]2–
