 Short Answer Type
Short Answer TypeWhy is [Cr(NH3)6]3+ ion not diamagnetic?
Electronic configuration of Cr is 4s1 3d5 . the oxidation number of Cr is in [Cr(NH3)6]3+ is Cr3+. therefore,
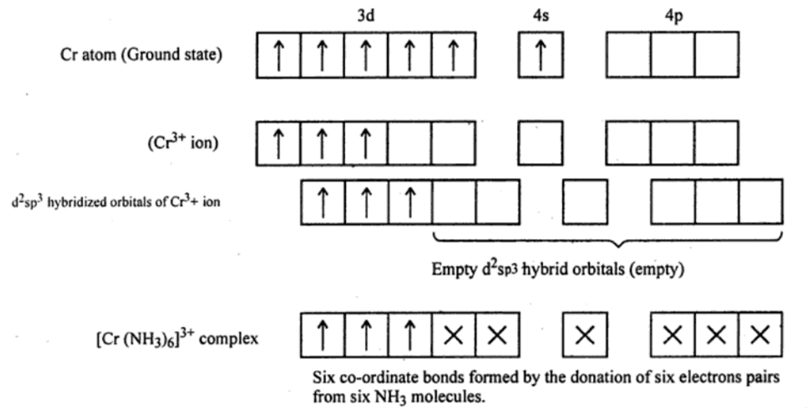
Six pairs of electrons, one from each NH3 molecule, occupy the six hybrid orbitals. Thus, the complex has octahedral geometry. due to unpaired electrons the complex is paramagnetic.
In a geometry of [PtCl4]2– is square planar, what orbitals of platinum are involved in the bonding?
