 Short Answer Type
Short Answer TypeWhat is the type of hybridization associated with Cu2+ ion in [Cu(NH3)4]2+ complex?
4- Coordinate complex will be tetrahedral or square planar. In complex [Cu(NH3)4]2+. According to VBT the complex will tetrahedral.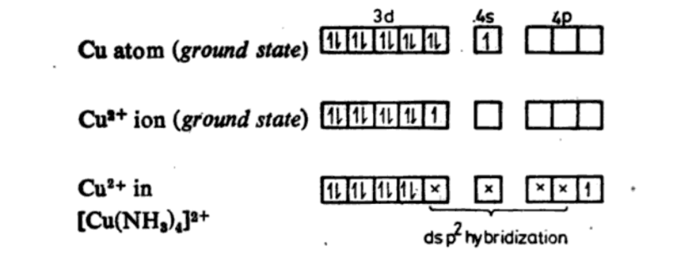
The hybrization of the complex is dsp2.
In a geometry of [PtCl4]2– is square planar, what orbitals of platinum are involved in the bonding?
