 Short Answer Type
Short Answer TypeIn a geometry of [PtCl4]2– is square planar, what orbitals of platinum are involved in the bonding?
Give an example of hexadentate ligand.
EDTA4- (ethylene diamine tetracetate ion) is hexadentate ligand as it can bind through two nitrogen and four oxygen atoms to central metal ion.
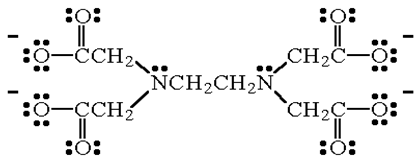
structure of EDTA4-
