 Short Answer Type
Short Answer TypeUsing valency bond approach predict the shape and magnetism (paramagnetic or diamagnetic) of [Ni(CN)4]–.
The valence shell electronic configuration of ground state Ni atom is 3d8 4s2.
All of these 10 electrons are pushed into 3d orbitals and get paired up when strong field CO ligands approach Ni atom. The empty 4s and three 4p orbitals undergo sp3 hybridization and form bonds with CO ligands to give Ni(CO)4. Thus Ni(CO)4 is diamagnetic.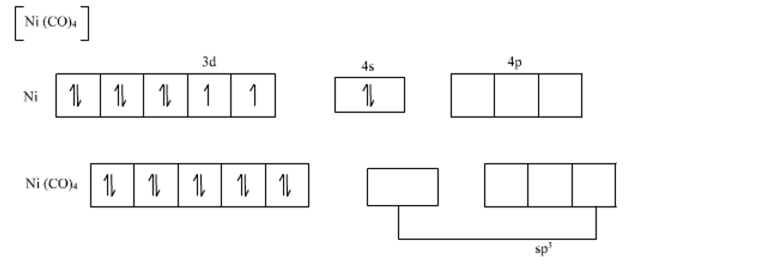
Since hybirdization of Ni(CO)4 is sp3 therefore according to VBT it has tetrahedral shape.
Explain the following terms: (i) Inner orbital complex and (ii) outer orbital complex.
Deduce the structures of [NiCl4]2– and [Ni(CN)4]2– considering the hybridization of the metal ion. Calculate the magnetic moment (spin only) of the species.
 Long Answer Type
Long Answer TypeIllustrate with an example each of the following terms: (i) Ionization isomerism, (ii) coordination isomerism, (iii) Linkage isomerism, (iv) Geometrical isomerism (v) Optical isomerism.
Draw the isomers of each of the following:
(i) [Pt(NH3)2Cl2] (ii) [PtCl3Br3]2–
(ii) [Co(NH3)4Cl2] (iv) [Co(en)2Cl2]+.
