 Short Answer Type
Short Answer TypeWrite the IUPAC name of the complex [Cr(NH3)4Cl2]+. What type of isomerism does it exhibit?
Write the IUPAC names of the following coordination compounds:
(i) [Cr(NH3)3Cl3]
(ii) K3[Fe(CN)6]
(iii) [CoBr2(en)2]+, (en = ethylenediamine)Give the formula of each of the following coordination entities:
(i) Co3+ Ion is bound to one Cl-1 , one NH3 molecule and two bidentate ethylenediamine (en) molecules.
(ii) Ni2+ ion is bound to two water molecules and two oxalate ions. Write the name and magnetic behavior of each of the above coordination entities.
(At. No. Co = 27, Ni = 28)
Write the name, stereochemistry and magnetic behavior of the following:
(At. nos. Mn = 25, Co = 27, Ni = 28)
(i) K4[Mn (CN)6]
(ii) [Co (NH3)5Cl]Cl2
(iii) K2[Ni (CN)4]
Using IUPAC norms write the formulate for the following coordination compounds:
(i) Hexaamminecobalt (III) chloride
(ii) Potassium tetrachloridonickelate (II)
(a) Write the hybridization and shape of the following complexes:
(i) [CoF6]3–
(ii) [Ni (CN)4]2–
(Atomic number: Co = 27, Ni = 28)
(b) Out of NH3 and CO, which ligand forms a more stable complex with a transition metal and why?
(a)
(i) The atomic number of Co is 27 and its valence shell electronic configuration is 3d74s2.
Co is in +3 oxidation state in the complex [CoF6]3-.

Hence, [CoF6]3- is sp3d2 hybridized and it is octahedral in shape.
(ii) The atomic number of Ni is 28 and its valence shell electronic configuration is 3d84s2.
Ni is in +2 oxidation state in the complex [Ni(CN)4]2–.
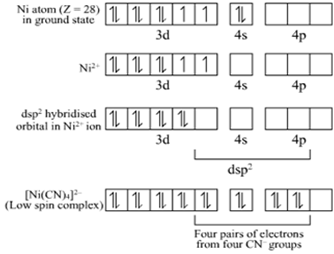
Hence, [Ni(CN)4]2– is dsp2 hybridized and it is square planar in shape.
(b) Out of NH3 and CO, CO ligand forms a more stable complex with transition metal because the metal- carbon bonds in metal carbonyls have both and characters .A bond is formed when carbonyl carbon donates a lone pair of electrons to the vacant orbital of the metal. A bond formed by the donation pair of electrons from filled metal d orbital into the vacant anti- bonding orbital ( also known as back bonding of the carbonyl group). And sigma bond strengthen the pi bond vice-versa. Thus, a synergic effect s created due to this metal- ligand bonding. These synergic effects strengthen the bond between CO and the metal.
For the complex [NiCl4]2-, write
(i) The IUPAC name.
(ii) The hybridization type.
(iii) The shape of the complex.
(Atomic no. of Ni = 28)
What is meant by crystal field splitting energy? On the basis of crystal field theory, write the electronic configuration of d4 in terms of t2g and eg in an octahedral field when
(i)![]() 0 > P
0 > P
(ii) ![]() 0 < P
0 < P
Name of the following coordination entities and describe their structure:
(i) [Fe (CN)6]4-
(ii) [Cr (NH3)4Cl2]+
(iii) [Ni (CN) 4]2-
(Atomic numbers Fe = 26. Cr = 24, Ni = 28