 Short Answer Type
Short Answer TypeWrite the hybridisation and number of unpaired electrons in the complex [CoF6]3-.
Atomic No. of Co = 27
Give reason:
When Cl2 reacts with an excess of F2. ClF3 is formed and not FCl3
Chlorine has empty d-orbital and it acquires excited state at the time of bonding when an electron from 3p-orbital are promoted to 3d- orbital.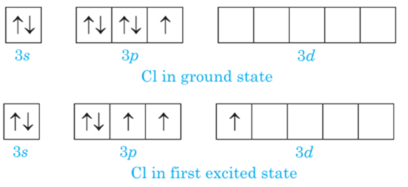
In first excited state chlorine atom can exhibit a covalency of three, hence cannot expand its octet due to the absence of empty d- orbitals in 2nd energy shell.
Hence, it cannot exhibit covalency more than 1therefore FCl3 is not possible.
 Multiple Choice Questions
Multiple Choice QuestionsThe number of unpaired electrons in[NiCl4]2-, Ni(CO)4 and (Cu(NH3)4]2+ respectively are
0, 2, 1
2, 0, 1
0, 2, 1
2, 2, 0
The pair having the same magnetic moment is:
[At. No. : Cr=24, Mn=25, Fe=26, Co=27]
[Cr(H2O)6 ]2+ and [Fe(H2O)6 ]2+
[Mn(H2O)6 ]2+ and [Cr(H2O)6 ]2+
[CoCl4 ]2− and [Fe(H2O)6 ]2+
[CoCl4 ]2− and [Fe(H2O)6 ]2+
