 Multiple Choice Questions
Multiple Choice QuestionsIn which of the following pairs are both the ions coloured in aqueous solution?
(At. no.: Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 2)
Ni2+, Ti3+
Sc3+, Ti3+
Sc3+,Co2+
Sc3+,Co2+
A.
Ni2+, Ti3+
Ni28 = 1s2s, 2s2, 2p6, 3s2,3p6,3d8,4s2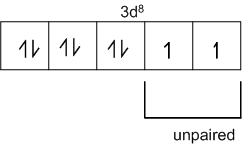
Ni2+ = 1s2, 2s2, 2p6, 3s2,3p6,3d8
Ti22 = 1s2,2s2, 2p6, 3s2,3p6,3d2,4s2
Which of the following is not chiral
2-butanol
2,3 -dibromo pentane
3- bromopentane
3- bromopentane
[Co(NH3)4(NO2)2]Cl exhibits:
linkage isomerism, ionisation isomerism and optical isomerism
Linkage isomerism, ionisation isomerism and geometrical isomerism
ionization isomerism, geometrical isomerism and optical isomerism
ionization isomerism, geometrical isomerism and optical isomerism
[Cr(H2O)6]Cl3 (at. no. of Cr = 24) has a magnetic moment of 3.83 BM, the correct distribution of 3d electrons in the chromium of the complex is:




The number of unpaired electrons in a paramagnetic diatomic molecule of an element with atomic number 6 is:
2
3
4
4
An example of a sigma bonded organometallic compound is :
Ruthenocene
Grignard's reagent
Ferrocene
Ferrocene
The correct order of the stoichiometries of AgCl formed when AgNO3 in excess is treated with the complexes: CoCl3.6NH3, CoCl3.5NH3, CoCl3.4NH3 respectively is
1 AgCl, 3 AgCl, 2 AgCl
3 AgCl, 1 AgCl, 2 AgCl
3 AgCl, 2 AgCl, 1 AgCl
3 AgCl, 2 AgCl, 1 AgCl
Correct increasing order for the wavelengths of absorption in the visible region for the complexes of Co3+ is
[Co(en)3]3+, [Co(NH3)6]3+, [Co(H2O)6]3+
[Co(H2O)6]3+, [Co(en)3]3+, [Co(NH3)6]3+
[Co(H2O)6]3+, [Co(NH3)6]3+, [Co(en)3]3+
[Co(H2O)6]3+, [Co(NH3)6]3+, [Co(en)3]3+
Pick out the correct statement with respect
[Mn(CN)6]3–
It is sp3d2 hybridised and octahedral
It is sp3d2 hybridised and tetrahedral
It is d2sp3 hybridised and octahedral
It is d2sp3 hybridised and octahedral
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code :
| Column I | Column II | ||
| a. | Co3+ | i. | |
| b. | Cr3+ | ii. | |
| c. | Fe3+ | iii. | |
| d. | Ni2+ | iv. | |
| v. | |||
| a | b | c | d |
| iv | v | ii | i |
| a | b | c | d |
| i | ii | iii | iv |
| a | b | c | d |
| iii | v | i | ii |
| a | b | c | d |
| iv | i | ii | iii |
