 Multiple Choice Questions
Multiple Choice QuestionsThe type of isomerism shown by the complex [CoCl2(en)2] is
Geometrical isomerism
Coordination isomerism
Linkage isomerism
Ionization isomerism
A.
Geometrical isomerism
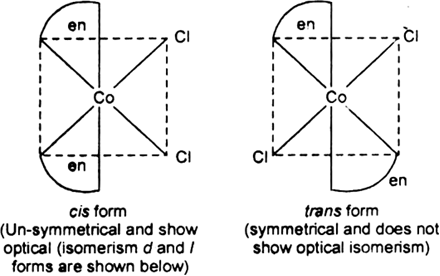
In the given complex, the CN of Co is 6, and the complex has octahedral geometry.
The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Square planar geometry and diamagnetic
Tetrahedral geometry and diamagnetic
Tetrahedral geometry and paramagnetic
Square planar geometry and paramagnetic
Consider the following species:
CN+, CN–, NO and CN
Which one of these will have the highest bond order?
NO
CN-
CN
CN+
Which of the following coordination compounds would exhibit optical isomerism?
Pentamminentirocobalt (III) iodide
Tris-(ethylenediamine) cobalt (III) bromide
Trans-dicyanobis (ethylenediamine)
Diamminedinitroplatinum (II)
Sulphur reacts with chlorine in 1:2 ratio and forms X. Hydrolysis of X gives a sulphur compound Y. The hybridization of the central atom in the anion Y is
sp3
sp2
sp3d
sp
In [CO(H2O)6]2+, there are three unpaired electrons present. The calculated is 3.87 BM which is quite different from the experimental of 4.40 BM. This is because of
increase in number of unpaired electrons
some contribution of the orbital motion of the electron to the magnetic moment
change in orbital spin of the electron
d-d transition
Anhydrous mixture of KF and HF contain which types of ions?
K+, H+, F-
{KF+, (HF-)}
KH+, F-
K+, H
