 Multiple Choice Questions
Multiple Choice QuestionsThe complex used as an anti-cancer agent is
mer-[Co(NH3)3Cl3]
cis-[PtCl2(NH3)2]
cis-K2[PtCl2Br2]
Na2[CoCl4]
Which of the following does not have optical isomer?
[Co(NH3)3Cl3]
[Co(en)3]Cl3
[Co(en)2Cl2]Cl
[Co(en)(NH3)2Cl2]Cl
Assertion : The [Ni(en)3]Cl2 (en = ethylene diamine) has lower stability than [Ni(NH3)6]Cl5.
Reason : In [Ni(en)3]Cl2 the geometry of Ni is trigonal bipyramidal.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
In the following compound, the number of sp-hybridised carbons are
CH2 = C = CH - CN(CH) - C ≡ CH
2
3
4
5
Which of the following molecule or ions is a bidentate ligand?
A.
Bidentate ligands are those ligand which can coordinate to metal atom by two donor atoms.
e.g.
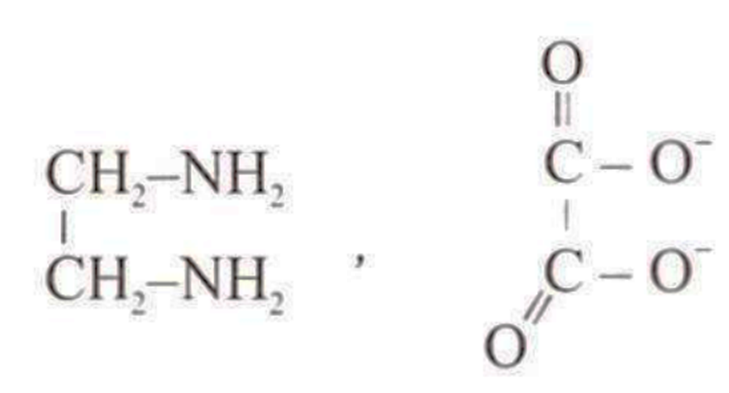
CO is practically non-polar since
the sigma electron drift from C to O is almost nullified by the pi-electron drift from O to C
the sigma electron drift from O to C is almost nullified by the pi-electron drift from C to O
the bond moment is low
there is a triple bond between C and O
What is the correct electronic configuration of the central atom in K4[Fe(CN)6] based on crystal field theory
