 Short Answer Type
Short Answer Type(a) Define molar conductivity of a solution and explain how molar conductivity changes with a change in concentration of solution for a weak and a strong electrolyte.
(b) The resistance of a conductivity cell containing 0.001 M KCl solution at 298 K is Ω 1500 . What is the cell constant if the conductivity of 0.001 M KCl solution at 298 K is 0.146x10-3 S cm-1?
Molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing 1 mole of the electrolyte kept between two electrodes with the area of cross-section A and distance of unit length.![]()
Molar conductivity increases with a decrease in concentration. This is because the total volume V of the solution containing one mole of the electrolyte increases on dilution.
Variation of molar conductivities with dilution:
For strong electrolytes, molar conductivity slowly increases with dilution.
For weak electrolytes, molar conductivity increases steeply on dilution, especially near lower concentrations. The variation of with for strong and weak electrolytes is shown in the following plot: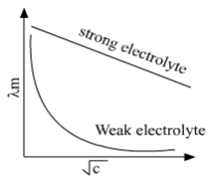
b) Given,
Conductivity, k = 0.146 x 10-3 S cm-1
Resistance, R = 1500 ohm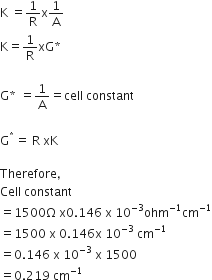
The chemistry of corrosion of iron is essentially an electrochemical phenomenon. Explain the reactions occurring during the corrosion of iron in the atmosphere.
Determine the values of equilibrium constant (KC) and  G° for the following reaction:
G° for the following reaction: 
The conductivity of 0.20 mol L-1 solution of KCl is 2.48 x 10-2 S cm-1. Calculate its molar conductivity and degree of dissociation (K+) = 73.56 S cm2 mol-1 and (Cl-)= 76.5 S
(b) What type of battery is mercury cell? Why is it more advantageous than dry cell?
The standard electrode potential (E°) for Daniell cell is +1·1 V. Calculate the G° for the reaction
Zn(s) + Cu2+ (aq) ----> Zn+ (aq) + Cu (s)
(1 F = 96500 C mol-1).
Calculate the emf of the following cell at 25°C:

Given E°cell = + 0.46 V and log 10n = n
