 Short Answer Type
Short Answer TypeExpress the relation among cell constant, the resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solution related to its conductivity?
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9S cm2mol-1. Calculate the conductivity of the conductivity of this solution.
The electrical resistance of a column of 0.05 M NaOH solution of diameter 1cm and length 50
cm is 5.55 x 103 ohm. Calculate its resistivity, conductivity and molar conductivity.
From the given cells:
Lead storage cell, Mercury cell, Fuel cell and Dry cell Ans the following:
(i) Which cell is used in hearing aids?
(ii) Which cell was used in Apollo Space Programme?
(iii)Which cell is used in automobiles and inverters?
(iv)Which cell does not have long life?
Calculate e.m.f of the following cell at 298 K:
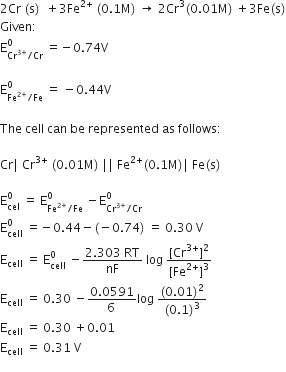
 Long Answer Type
Long Answer Type(a) What type of a battery is lead storage battery? Write the anode and cathode reactions and the overall cell reaction occurring in the operation of a lead storage battery.
(b) Calculate the potential for half-cell containing 0.10 M K2Cr2O7 (aq), 0.20 M Cr3+(aq) and 1.0 x 10-4 M H+ (aq)
The half-cell reaction is 
And the standard electrode potential is given as E0 = 1.33 V.
OR
(a) How many moles of mercury will be produced by electrolysing 1.0 M?
Hg (NO3)2 solution with a current of 2.00 A for 3 hours?
[Hg (NO3)2 = 200.6 g mol-1]
(b) A voltaic cell is set up at 25°C with the following half-cells Al3+ (0.001 M) and Ni2+ (0.50 M). Write an equation for the reaction that occurs when the cell generates an electric current and determine the cell potential.

 Short Answer Type
Short Answer TypeState Kohlrausch's law of independent migration of ions. Why does the conductivity of a solution decrease with dilution?
(a) Calculate  for the reaction
for the reaction
Mg (s) + Cu2+ (aq) → Mg2+ (aq) + Cu (s)
Given : E°cell = + 2.71 V, 1 F = 96500 C mol−1
(b) Name the type of cell which was used in Apollo space programme for providing electrical power.
Calculate the degree of dissociation (a) of acetic acid if its molar conductivity (Λm) is 39.05 S cm2mol–1. Given λo(H+) = 349.6 S cm2 mol–1 and λo(CH3COO–) = 40.9 S cm2 mol–1
