 Multiple Choice Questions
Multiple Choice Questions| Electrolyte | KCl | KNO3 | HCl | NaOAc | NaCl |
| ∧∞S cm2 mol- | 149.9 | 145.0 | 426.2 | 91.0 | 126.5 |
517.2
552.7
390.7
390.7
Calomel (Hg2Cl2) on reaction with ammonium hydroxide gives
HgNH2Cl
NH2 – Hg – Hg – Cl
Hg2O
Hg2O
Which among the following factors is the most important in making fluorine the strongest oxidizing halogen?
Electron affinity
Bond dissociation energy
Hydration enthalpy
Hydration enthalpy
In hydrogen-oxygen fuel cell, combustion of hydrogen occurs to
generate heat
remove adsorbed oxygen from electrode surfaces
produce high purity water
produce high purity water
D.
produce high purity water
Any cell (such as fuel cell), works when a potential difference is developed.
Consider the following E° values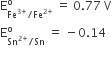
Under standard conditions the potential for the reaction
Sn(s) + 2Fe3+(aq) → 2Fe2+(aq) + Sn2+(aq) is
1.68 V
0.63 V
0.91 V
0.91 V
The standard e.m.f of a cell, involving one electron change is found to be 0.591 V at 25°C. The equilibrium constant of the reaction is (F = 96,500 C mol-1: R = 8.314 JK-1 mol-1)
1.0×101
1.0×1030
1.0×1030
1.0×1030
The limiting molar conductivities Λ° for NaCl, KBr and KCl are 126, 152 and 150 S cm2 mol-1 respectively. The Λ° for NaBr is
128 S cm2 mol-1
302 S cm2 mol-1
278 S cm2 mol-1
278 S cm2 mol-1
In a cell that utilises the reaction Zn(s) + 2H+ (aq) → Zn2+(aq) + H2(g) addition of H2SO4 to cathode compartment, will
lower the E and shift equilibrium to the left
increases the E and shift equilibrium to the left
increase the E and shift equilibrium to the right
increase the E and shift equilibrium to the right
The 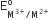 values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
Cr
Co
Fe
Fe
How long (approximate) should water be electrolysed by passing through 100 amperes current so that the oxygen released can completely burn 27.66 g of diborane? (Atomic weight of B = 10.8 u)
1.6 hours
6.4 hours
0.8 hours
3.2 hours
