 Multiple Choice Questions
Multiple Choice QuestionsThe standard emf of galvanic cell involving 3 moles of electrons in its redox reaction is 0.59 V. The equilibrium constant for the reaction of the cell is
1025
1020
1015
1030
E1, E2 and E3 are the emfs of the following three galvanic cells respectively.
(i) Zn (s) | Zn2+ (0.1 M) || Cu2+ (1M) | Cu (s)
(ii) Zn (s) | Zn2+ (1 M) || Cu2+ (1M) | Cu (s)
(iii) Zn (s) | Zn2+ (1 M) || Cu2+ (0.1M) | Cu (s)
Which one of the following is true?
E2 > E1 > E3
E1 > E2 > E3
E3 > E1 > E2
E3 > E2 > E1
The standard emf of a galvanic cell involving 2 moles of electrons in its redox reaction is 0.59 V. The equilibrium constant for the redox reaction of the cell is
1020
105
10
1010
9.65 C of electric current is passed through fused alyhdrous MgCl2. The magnesium metal thus obtained is completely converted into a Grignard reagent. The number of moles of Grignard reagent obtained is
5 × 10-4
1 × 10-4
5 × 10-5
1 × 10-5
E1, E2, E3 are the emf values of the three galvanic cells respectively.
Which one of the following is true?
E1> E2 >E3
E2 >E3 >E1
E3 >E2 > E1
E1> E3 >E2
Which one of the following has a potential more than zero?
Pt, H2 (1 atm) | HCl (2M)
Pt, H2 (1 atm) | HCl (0.1 M)
Pt, H2 (1 atm) | HCl (0.5 M)
Pt, H2 (1 atm) | HCl (1M)
100 mL of 0.1 M acetic acid is completely neutralized using a standard solution of NaOH. The volume of ethane obtained at STP after the complete electrolysis of the resulting solution is
56 mL
224 mL
560 mL
112 mL
D.
112 mL
Acetic acid on electrolysis gives ethane as
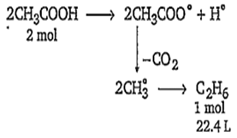
Given moles of acetic acid,
CH3COOH = = 0.01
2 mol CH3COOH gives C2H6 = 22.4 L
0.01 mol CH3COOH will give
C2H6 =
= 0.112 L
= 112 mL
The emf of a galvanic cell constituted with the electrode Zn2+|Zn (-0.76V) and Fe2+|Fe (-0.41V) is
-0.35V
+ 1.17V
+0.35V
-1.17V
For hydrogen-oxygen fuel cell at 1 atm and 298 K
H2 (g) + O2 (g) → H2O (l) ; G° = -240 kJ
E° for the cell is approximately, (Given F = 96500 C)
2.48 V
1.25 V
2.5 V
1.26 V
While charging the lead storage battery,
PbSO4 on anode is reduced to Pb
PbSO4 on cathode is reduced to Pb
PbSO4 on cathode is oxidised to Pb
PbSO4 on anode is oxidised to PbO2
