 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss pollution caused by CO.
Or
What are different sources of CO pollution? What are the effects of continuous exposure to CO on human beings?
 Short Answer Type
Short Answer TypeExplain giving reasons: There presence of CO reduces the amount of haemoglobin available in the blood for carrying oxygen to the body cells.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss sources and sinks of CO2 in the atmosphere.
Or
Discuss the role of CO2 as a pollutant.
Or
Carbon dioxide is inert and harmless gas, yet it is considered to be a serious pollutant. Explain.
Discuss air pollution caused by oxides of nitrogen.
Or
What are the principal environmental effects of NO2?
Or
Name the oxide of nitrogen present in the atmosphere. What are the sources and sinks of NOx?
A number of oxides of nitrogen such as NO, N2O, NO2, N2O3 and N2O5 are introduced into the atmosphere due to the natural sources and due to human activity. NO and NO2 are considered as pollutants and denoted by the general formula NOx.
The source of NOx. Nitric oxide (NO) is a colourless gas and nitrogen dioxide (NO2) is reddish brown gas having a pungent smell and is suffocating in nature.
(i) Natural sources: Natural bacterial action is the only natural source which discharges NOX mainly in the form of NO into the atmosphere in large quantity. Lightning discharge also results in the combination of N2 and O2 to form NO.
(ii) Man-made sources: The major man-made sources of NOx are combustion of coal, oil, natural gas and gasoline. The basic reactions are: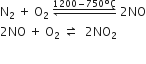
(iii) Chemical industries as a source: Chemical industries like sulphuric acid and nitric acid industries produce NOx as by-products which are discharged into the air.
Sinks of NOx: NOx (i.e. NO and NO2) in the atmosphere are converted into nitric acid through the following reactions in which ozone also takes part: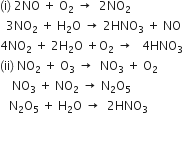
Nitric acid acts as a temporary sink and comes down in the form of acid rain or precipitates as nitrate salts after reacting with bases such as ammonia, lime etc.
Harmful effects of NOx pollution:
(i) Nitric oxide binds to haemoglobin and decreases oxygen transport efficiency of blood.
(ii) Acid rain (HNO3) can cause the pH of the water to drop to 4 or 5. This can affect vegetation and building materials.
(iii) The sunlight reacts with nitrogen dioxide to produce highly active oxygen atoms.
The active oxygen immediately reacts with traces of hydrocarbons in the air and produces irritates called photochemical smog. This is a health hazard.
(iv) Oxides of nitrogen have a harmful effect on nylon, rayon and cotton yarns and also cause of fading of dyes used for textiles.
(v) Nitrogen dioxide (NO2) results in respiratory problems in human beings and leads to bronchitis.
How does SO2 cause pollution?
Or
What are the principal environmental effects of SO2?
Or
Describe sources, sinks and polluting effects of SO2.
