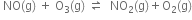Short Answer Type
Short Answer TypeWrite the expression for the equilibrium constant Kc for each of the following reactions:![]()
Write the expression for the equilibrium constant Kc for each of the following reactions:
Write the expression for the equilibrium constant Kc for each of the following reactions:![]()
Write the expression for the equilibrium constant Kc for each of the following reactions:![]()
 Long Answer Type
Long Answer TypeIt has been found that the pH of a 0.01 M solution of an organic acid is 4.15. Calculate the concentration of the anion, the ionisation constant of the acid and its pKa.
 Short Answer Type
Short Answer TypeThe concentration of pure liquids and solids in the equilibrium expression for heterogeneous reactions are ignored because their concentrations remain constant. By convention, the concentration of all pure solids and pure liquids is taken as unity, i.e.
[solid] = 1, [liquid] = 1.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type