 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeThe reversible reaction is,
В В В В В 
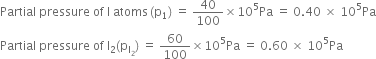
Applying the law of chemical equilibrium
В В В В В В В В В В В 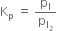
Putting the values, we have,
В В В В В В 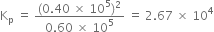
В В
 Short Answer Type
Short Answer TypeThe value of Kc for the reactionВ ![]() В isВ
В isВ ![]() В atВ
В atВ ![]() В If the equilibrium concentration ofВ
В If the equilibrium concentration ofВ ![]() В in air atВ
В in air atВ ![]() В isВ
В isВ ![]() В what is the concentration of O3?vВ
В what is the concentration of O3?vВ
 Long Answer Type
Long Answer TypeCalculate the value of equilibrium constant for the reaction:![]()
There is 10.0 mol ofВ N2, 14В·0 mol of O2В and 0В·2 mol of NO2В present at equilibrium in a 3В·0L vessel at 298K.What will be the effect of increased temperature on the equilibrium constant?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type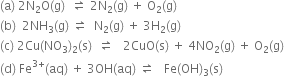
 Long Answer Type
Long Answer Type