 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe value of Kc for the reaction ![]() is
is ![]() at
at ![]() If the equilibrium concentration of
If the equilibrium concentration of ![]() in air at
in air at ![]() is
is ![]() what is the concentration of O3?v
what is the concentration of O3?v
 Long Answer Type
Long Answer TypeCalculate the value of equilibrium constant for the reaction:![]()
There is 10.0 mol of N2, 14·0 mol of O2 and 0·2 mol of NO2 present at equilibrium in a 3·0L vessel at 298K.What will be the effect of increased temperature on the equilibrium constant?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type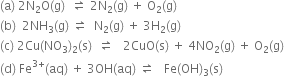
 Long Answer Type
Long Answer TypeState and explain Le-Chatelier’s principle.
Le-Chatelier’s principle. This principle may be stated as if a stress (such as a change in concentration, temperature or pressure) is applied to a system in equilibrium, the equilibrium shifts in a way to undo or nullify the effect of the imposed stress.
(i) Effect of change of concentration on equilibrium. If the concentration of any one or all the reactants is increased, the equilibrium shifts towards right hand side to form more products whereas increase in the concentration of any one or all the products shifts the equilibrium towards left hand side to form more reactants in order to nullify the effect of increase in the concentration of reactants or products respectively. For example, consider the reaction,
Increase in concentration of reactants (N2, H2) will shift the equilibrium in the forward direction in order to decrease their concentration. The addition of extra NH3 from outside the equilibrium mixture will shift the equilibrium in the backward direction.
(ii) Effect of temperature on equilibrium. According to Le-Chatelier’s principle of increasing the temperature, the equilibrium shifts towards that direction where absorption of heat (endothermic change) takes place in order to nullify the effect of the rise in temperature. On the other hand, on decreasing the temperature, the equilibrium shifts towards that direction where the evolution of heat (exothermic change) takes place in order to nullify the effect of a decrease in temperature. For example.

On decreasing the temperature, the equilibrium shifts in the forward direction i.e. towards the exothermic reaction (evolution of heat). Thus, a decrease in temperature favours the formation of sulphur trioxide.
On increasing the temperature, the equilibrium shifts in the forward direction i.e. towards the endothermic reaction (absorption of heat). Thus, an increase in the temperature favours the formation of nitric oxide.
(iii) Effect of change in pressure on equilibrium. On increasing the pressure, the number of moles per unit volume increases and thus according to Le-Chatelier’s principle the equilibrium shifts towards the side where the number of moles per unit volume decreases in order to nullify the effect of an increase in pressure. For example.
On increasing the pressure, the number of moles per unit volume increases and thus according to Le-Chaterlier's principle the equilibrium shifts towards the right-hand side (i.e. towards the formation of ammonia) where the number of moles per unit volume decreases.
(iv) Effect of the catalyst. A catalyst has no effect on equilibrium point. This is because it increases the rate of the forward as well as backward reaction to the same extent. Thus, a catalyst does not affect the position of equilibrium, but simply helps to achieve the equilibrium in a shorter time i.e. quickly.
