 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe value of Kc for the reaction ![]() is
is ![]() at
at ![]() If the equilibrium concentration of
If the equilibrium concentration of ![]() in air at
in air at ![]() is
is ![]() what is the concentration of O3?v
what is the concentration of O3?v
 Long Answer Type
Long Answer TypeCalculate the value of equilibrium constant for the reaction:![]()
There is 10.0 mol of N2, 14·0 mol of O2 and 0·2 mol of NO2 present at equilibrium in a 3·0L vessel at 298K.What will be the effect of increased temperature on the equilibrium constant?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type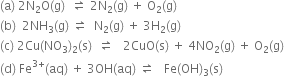
 Long Answer Type
Long Answer Type
(i) Effect of concentration. Increase in concentration of reactants (N2, H2) will shift the equilibrium in the forward direction to form more ammonia in order to decrease their concentrations. The addition of extra NH3 from outside to the equilibrium mixture will shift the equilibrium in the backward direction. Thus, the addition of N2 and H2 favours the formation of ammonia.
(ii) Effect of temperature. The forward reaction is exothermic in nature while the backward reaction is endothermic in nature. According to Le-Chatelier’s principle, on decreasing the temperature, the equilibrium shifts towards that direction where the evolution of heat takes place in order to nullify the effect of decreasing temperature. Thus, a decrease in temperature favours the formation of ammonia.
On the other hand, on increasing the temperature the equilibrium shifts towards the backward direction where absorption of heat takes place in order to nullify the effect of the rise in temperature. Thus, lower temperature favours the formation of ammonia.
(iii) Effect of pressure. On increasing the pressure, the number of moles per unit volume increases and thus according to Le-Chatelier’s principle, the equilibrium shifts towards that side where the number of moles per unit volume decreases in order to nullify the effect of an increase in pressure. On the other hand, on decreasing the pressure, the equilibrium shifts towards the backwards direction i.e. ammonia decomposes to give N2 and H2. Thus, an increase in pressure favours the formation of ammonia.
Hence favourable conditions in the formation of ammonia are:
(i) The addition of N2 and H2
(ii) Lower temperature
(iii) Higher pressure.
