 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss the ionisation of weak electrolytes (Ostwald’s dilution law).
 H+(aq) + A-(aq)
H+(aq) + A-(aq)
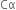
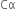
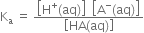
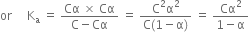
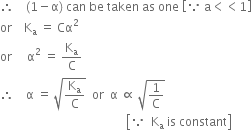
Thus, the degree of ionisation of a weak electrolyte is inversely proportional to the square root of the molar concentration of the solution of the electrolyte.
Hence the degree of ionisation of weak electrolyte goes on increasing with the decrease in molar concentration i.e. with dilution and it reaches the maximum value (unity) in very dilute solution. Thus, all weak electrolytes undergo almost complete ionisation at infinite dilution.
For a weak base BOH:
Let us consider a weak base BOH dissolved in water, a be the degree of ionisation and C be the molar concentration of BOH.

Initial conc. C 0 0
Equilibrium 


conc.
Applying law of chemical equilibrium,
 Short Answer Type
Short Answer TypeWrite the correct balanced net ionic equation for the reaction whose equilibrium constant at 298K is:
(i) Ka(C6H5COOH) = 6·3 × 10–5
(ii) Ka(H2C2O4) = 5·4 × 10–2
(iii) Ka ( HSO3–) = 2·8 × 10–7
(iv) Kb(OCl–) = 9·1 × 10–7
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type