 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss the Lowry Bronsted concept of acids and bases.
Or
Discuss the protonic concept of acids and bases.
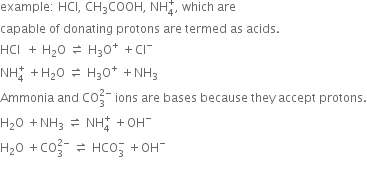

 Short Answer Type
Short Answer TypeState the formula and name of conjugate acid of the following bases:
(i) ![]() (ii) NH3
(ii) NH3
(iii) CH3COO– (iv) HS–
