 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeJustify the statement: All Bronsted bases are Lewis bases, but all Bronsted acids are not Lewis acids.
 Short Answer Type
Short Answer TypeOut of Lowry Bronsted concept and Lewis concept, which is regarded as better and why?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe ionic product of water is 0·11 × 10–14 at 273 K; 1·0 × 10–14 at 298K and 51 × 10–14 at 373K. Deduce from this data whether the ionisation of water into hydrogen and hydroxide ion is exothermic or endothermic.
 Long Answer Type
Long Answer TypeComment on the statement: An acidic solution contains OH– ions and even a basic solution contains H3O+ ions.
Or
How the values of Kw, [H3O+] and [OH–] are affected if acid or base is added to pure water at 298K?




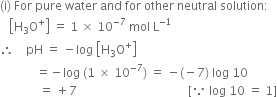
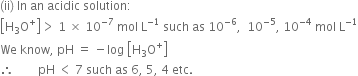
As pH of a solution goes on decreasing below 7, the acidic nature of solution goes on increasing.
If pH lies between:
(a) 0 and 2, the solution is strongly acidic,
(b) 2 and 4, the solution is moderately acidic,
(c) 4 and 7, the solution is weakly acidic.
(iii) In basic solution:

As pH of a solution goes on increasing above 7, the basic nature of the solution goes on increasing.
If pH lies between:
(a) 7 and 10, the solution is weakly basic,
(b) 10 and 12, the solution is moderately basic,
(c) 12 and 14, the solution is strongly basic.
 Short Answer Type
Short Answer Type