 Short Answer Type
Short Answer TypeThe pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ion in it.
What is the pH of an aqueous solution with hydrogen ion concentration equal to 3 × 10–5.mol L–l ? Is the solution acidic, basic or neutral?
 Long Answer Type
Long Answer Type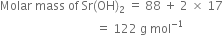
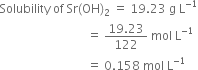
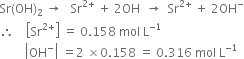
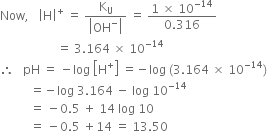
Calculate the pH of the following solutions:
(a) 2g of TIOH dissolved in water to give 2 litres of solution.
(b) 0.3g of NaOH dissolved in water to give 200 mL of solution.
 Short Answer Type
Short Answer TypeCalculate the pH of 1 ml of 13.6 MHCl diluted with water to give 1 litre of the solution.
 Long Answer Type
Long Answer TypeIf 0.561 KOH is dissolved in water to give 200 mL, of the solution at 298 K, calculate the concentration of potassium, hydrogen and hydroxyl ions. What is its pH ?
Calculate the pH of the resultant mixtures:
(a) 10 mL of 0·2 M Ca(OH)2 + 25 mL of 0·1M HCl
(b) 10 mL of 0·01 M H2SO4 + 10mLof 0·01 IM Ca(OH)2
(c) 10mL of 0·1 M H2SO4 + 10mL of 0·1 M KOH
