 Long Answer Type
Long Answer TypeHow many grammes of NaOH must be dissolved in one litre of the solution to give it a pH value of 12?
Calculate the pH of a solution obtained by mixing 150 mL of 0.1 N - NaOH and 150 ml of 0.2 N - HCl.
How does Arrhenius theory help in comparing the relative strengths of weak acids and weak bases?
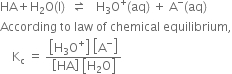
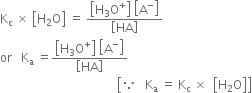
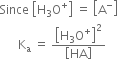

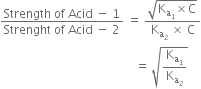
Hence the relative strength of two acids having the same molar concentration in aqueous solution may be compared in terms of the square root of their dissociation constants.
Relative strength of weak base:
The dissociation of weak base, say BOH may be represented as,
According to law of chemical equilibrium

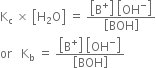
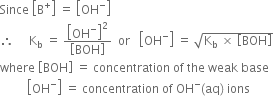

 Short Answer Type
Short Answer TypeArrange the following in decreasing order of acidic strength:
(i) HOCl (Ka = 3·0 × 10–8)
(ii) HCN (Ka = 4·0 × 10–10)
(iii) HNO3 (Ka = 4·5 × 10–4)
(iv) HF (Ka = 6·7 × 10–4)
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeIt has been found that the pH of a 0.01 M solution of an organic acid is 4.15. Calculate the concentration of the anion, the ionisation constant of the acid and its pKa.
