 Long Answer Type
Long Answer TypeHow many grammes of NaOH must be dissolved in one litre of the solution to give it a pH value of 12?
Calculate the pH of a solution obtained by mixing 150 mL of 0.1 N - NaOH and 150 ml of 0.2 N - HCl.
How does Arrhenius theory help in comparing the relative strengths of weak acids and weak bases?
 Short Answer Type
Short Answer TypeArrange the following in decreasing order of acidic strength:
(i) HOCl (Ka = 3·0 × 10–8)
(ii) HCN (Ka = 4·0 × 10–10)
(iii) HNO3 (Ka = 4·5 × 10–4)
(iv) HF (Ka = 6·7 × 10–4)
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type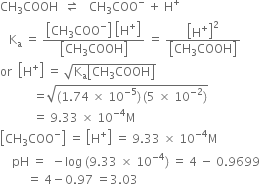
It has been found that the pH of a 0.01 M solution of an organic acid is 4.15. Calculate the concentration of the anion, the ionisation constant of the acid and its pKa.
