 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type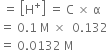
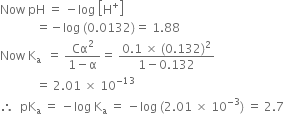
 Long Answer Type
Long Answer TypeAt 298 K, calculate the pH of 0.200 M solution of methylamine, CH3NH2(ionisation constant = 4·4×105).
 Short Answer Type
Short Answer TypeThe pH of 0.1 M solution of cyanic acid (HCNO) is 2.34. Calculate the ionisation constant of the acid and its degree of ionisation in the solution.
 Long Answer Type
Long Answer TypeThe ionisation constant of propanoic acid is 1·32 × 10–5. Calculate the degree of ionisation of the acid in its 0·05M solution and also it's pH. What will be its degree of ionisation if the solution is 0·01M in HCl also?
 Short Answer Type
Short Answer TypeThe pH of 0.1 M monobasic acid is 4.50. Calculate the concentration of species H+, A– and HA at equilibrium. Also, determine the values of Ka and pKa of the monobasic acid. A and HA at equilibrium. Also, determine the values of Ka and pKa of the monobasic acid.
