 Long Answer Type
Long Answer TypeShow that degree of hydrolysis of a salt of weak acid and weak base is independent of concentration of the solution.
Or
Derive the relation, ![]() in case of hydrolysis of a salt of weak acid and weak base.
in case of hydrolysis of a salt of weak acid and weak base.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeThe ionisation constant of nitrous acid is 4·5 × 10–4. Calculate the pH of 0·04M sodium nitrite solution and also its degree of hydrolysis.
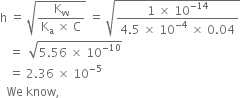
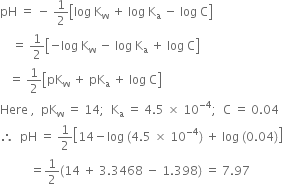
 Short Answer Type
Short Answer TypeThe pKa of acetic acid and pKb of ammonium hydroxide are 4·76 and 4·75 respectively. Calculate the pH of ammonium acetate solution.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type