 Multiple Choice Questions
Multiple Choice QuestionsIf H+ concentration of fruit juice is 3.3 × 10-2, then its pH will be
4.8 basic
4.8 neutral
4.8 acidic
1.6 acidic
The correct order of the acidity of following acids is-
CH3COOH, HCOOH, Cl-CH2COOH, F-CH2COOH, (Cl2)CHCOOH
Cl2CHCOOH > F-CH2COOH > Cl-CH2COOH > HCOOH > CH3COOH
F-CH2COOH > Cl-CH2COOH > Cl2CHCOOH > HCOOH > CH3COOH
HCOOH > CH3COOH > Cl-CH2COOH > Cl2CHCOOH > F-CH2COOH
None of the above
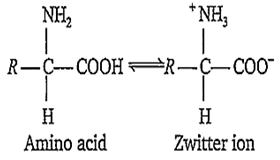
Zwitter ion is
a dipolar ion
ion formed from amino acid
internal salt
All of the above
D.
All of the above
The dipolar structure is commonly known as internal salt or zwitter ion.
Which of the following statement is correct?
Basic nature increases on increasing pH
Basic nature decreases on increasing pH
Acidic nature increases on increasing pH
None of the above
Which one of the following is conjugate acid of water in the reaction?
H2SO4 + H2O H3O+ + HSO
H2O
H3O+
SO
HSO
