 Long Answer Type
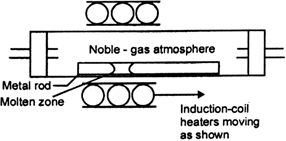
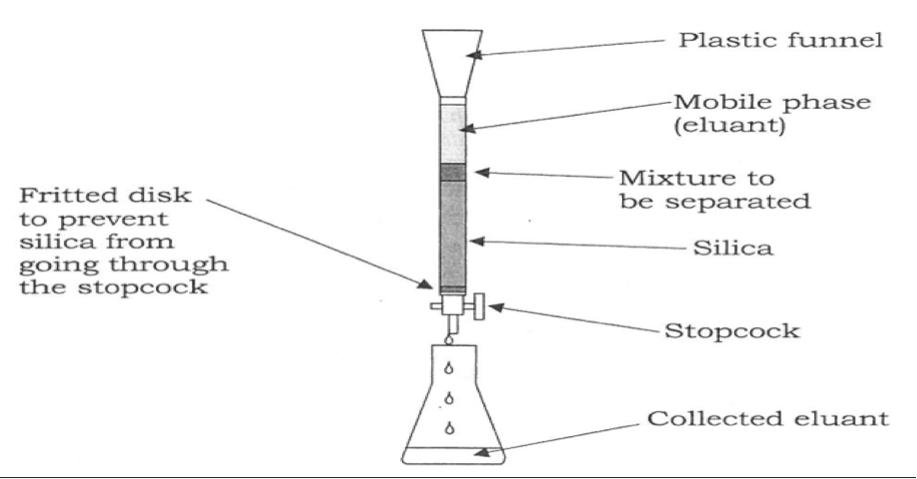
Long Answer TypeExplain: (i) Zone refining (ii) Column chromatography.
Write down the reactions taking place in different Zones in the blast furnance during the extraction of Iron.
 Short Answer Type
Short Answer TypeHow can you separate alumina from silica in a baxuite ore associated with silica? Give equations, if any.
 Long Answer Type
Long Answer TypeName the processes from which chlorine is obtained as a bye-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Outline the principles of refining of metals by the following methods:
(i) Zone refining.
(ii) Electrolytic refining.
(iii) Vapour phase refining.
