 Long Answer Type
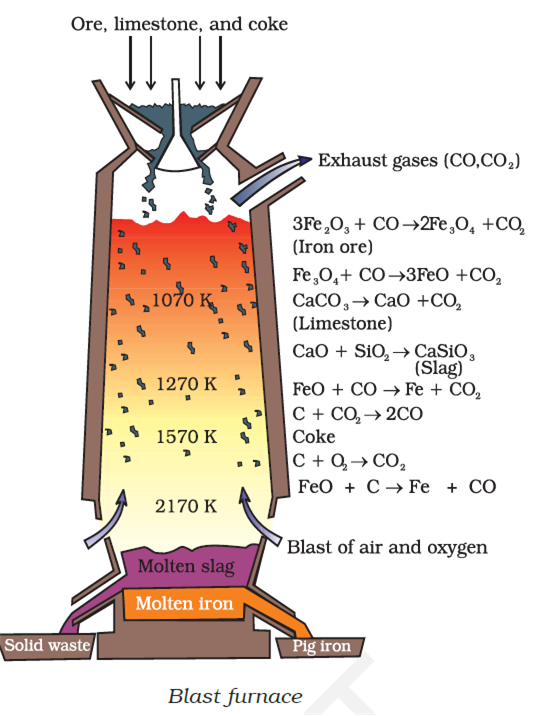
Long Answer TypeWrite down the reactions taking place in different Zones in the blast furnance during the extraction of Iron.
The higher temperature range, depend on the points of corresponding intersections in the ΔrG0 vs T plots. (Given in ncert book)
These reactions are as follows:
At 500 – 800 K (lower temperature range in the blast furnace)–
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4 CO2
Fe2O3 + CO → 2FeO + CO2
At 900 – 1500 K (higher temperature range in the blast furnace):
C + CO2 → 2CO
FeO + CO → Fe + CO2
The silicate impurity of the ore is removed as slag by calcium oxide (CaO), which is formed by the decomposition of limestone (CaCO3).
CaCO3 → CaO +CO2
CaO +SiO2 → CaSiO3
flux impurtiy (slag)
 Short Answer Type
Short Answer TypeHow can you separate alumina from silica in a baxuite ore associated with silica? Give equations, if any.
 Long Answer Type
Long Answer TypeName the processes from which chlorine is obtained as a bye-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Outline the principles of refining of metals by the following methods:
(i) Zone refining.
(ii) Electrolytic refining.
(iii) Vapour phase refining.
