 Long Answer Type
Long Answer TypeName the chief forms of the occurrence of the following in the earth crust:
(a) aluminium, (b) calcium, (c) sodium, (d) lead.
 Short Answer Type
Short Answer TypeSodium metal cannot be obtained by the electrolysis of aqueous sodium chloride solution.
Write the chemical reactions involved in the extraction of metallic silver from argentite.
Silver ores and native gold have to be leached with metal cyanides. Suggest a reason for this.
 Long Answer Type
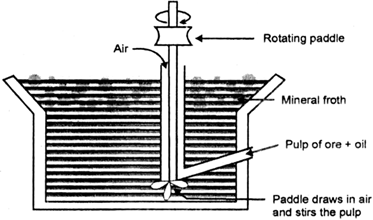
Long Answer TypeDescribe the principle of froth floatations process. What is the role of stabilizer and of a depressant? Give one example of each.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeState briefly the principles which serve as basis for the following operations in metallurgy:
(i) Froth floation process.
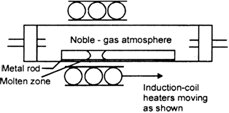
(ii) Zone refinnting.
(iii) Refining by liquation.
What chemical principle is involved in choosing a reducing agent for gettting the metal from its oxide ore? Consider the metal oxides, Al2O3 and Fe2O3 and justify the choice of reducing agent in each case.
