Short Answer Type
Short Answer TypeWhat is the function of anhydrous zinc chloride in the reaction between alcohols and hydrogen halide?
What is the difference in the molar masses of alkyl halides and corresponding parent haloalkanes?
Which will have higher boiling point CH3—CH2—CH2—CH2—Br or CH3—CH(CI)— CH3? Give reason.
 One Word Answers
One Word AnswersName the product formed when benzene diazonium chloride solution is warmed with aqueous KI.
 Short Answer Type
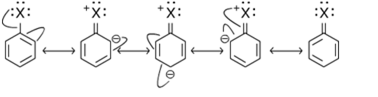
Short Answer TypeIn haloarenes the lone pair of electron on halogen atom is delocalized on the benzene ring. Since aryl halides are stabilizes by resonance hence the energy of acitvation for displacement of halogen form aryl halides is much greater than alkyl halides thus they not under go nucleophillic substiution reaction.