 Short Answer Type
Short Answer TypeWhich compound in each of the following pairs will react faster in SN2 reaction with HO–?
(a) CH3—Br or CH3I
(b) (CH3)3 Cl or CH3Cl2
(c) CH2=CHBr or CH2 = CH—CH2Br.
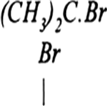
(ii) CH3CHCH2CH3
(iii) CH3CH2CH2CH2Br
(iv) (CH3)3 C—Cl
In the given compound, there are two different sets of equivalent b -hydrogen atoms. Thus, dehydrohalogenation of the compound yields two alkenes.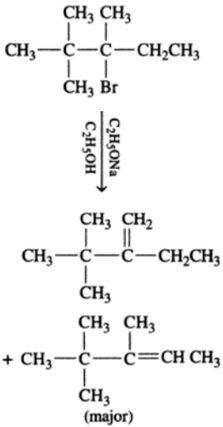
According to saytzeff’s rule in dehydrohalogenation reaction, the alkene having a greater number of alkyl groups attached to the doubly bonded carbon atom is preferably formed. Hence, alkene 3,4,4-trimethylpent-2-ene is the major product in this reaction.
