 Short Answer Type
Short Answer TypeIn the following pairs of halogen compounds which is faster undergoing SN2 reaction?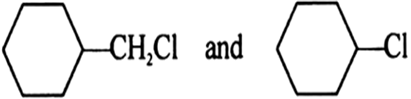
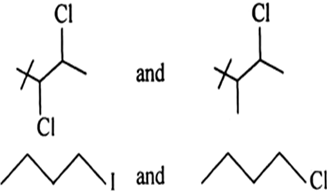
As there will be lesser steric hind– rance for the approaching nucleo– phile from the back side in (i).
As iodine is a better leaving group because of its large size, it will be released at a faster rate in the presence of incoming nucleophile.
 Long Answer Type
Long Answer TypePredict the order of reactivity of the following compounds in SN1 and SN2 reactions:
(a) The four isomeric bromobutanes.
(b) C6H5 H2Br, C6H5CH (C6H5Br, C6H5CH (CH3)Br, C6H5C(CH3)(C6H5)Br.
 Short Answer Type
Short Answer TypeWhy (-NO2) group shows its effect only at ortho- and para-position and not at meta–position?
 Long Answer Type
Long Answer Type Short Answer Type
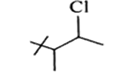
Short Answer TypeExplain the reason:
The reactivity order of alkyl bromides is tert alkyl bromide > sec alkyl bromide > primary alkyl bromide.
