 Short Answer Type
Short Answer TypeAn organic compound ‘A’ having the molecular formula C4H8 on treatment with dilute sulphuric acid give another compound ‘B’. B on treatment with conc. HCl and anhydrous zinc chloride gives ‘C’. C on treatment with sodium ethoxide gives back ‘A’. Identify the compound. Write the equations involved.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeExplain giving reason although haloalkanes are polar in character, yet they are insoluble in water.
Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene.
(i) 1-Bromo-1-methyl cyclohexane.
(ii) 2-Chloro-2-methyl butane.
(iii) 2, 2, 3-Trimethyl-3-bromopentane.
i) In 1-bromo-1-methylcyclohexane compound, all  atoms are equivalent. Thus, dehydrohalogenation of this compound gives only one alkene.
atoms are equivalent. Thus, dehydrohalogenation of this compound gives only one alkene.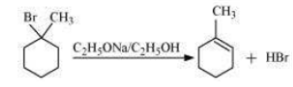
ii) In 2-chloro- 2-methyl butane compound, there are two different sets of equivalent  atoms. Thus, dehydrohalogenation of the compound yeilds two alkenes.
atoms. Thus, dehydrohalogenation of the compound yeilds two alkenes.
Saytzeff’s rule implies that in dehydrohalgenation reaction, the alkene having a greater number of alkyl groups attached to a doubly bonded carbon atoms is perferably produced. Hence, alkene (I) is the major product in this reaction.
iii) In the 2, 2, 3-Trimethyl-3 bromopentane compounds, there are two different  atoms. Thus, dehydrohalgenation of the compounds yields two alkenes.
atoms. Thus, dehydrohalgenation of the compounds yields two alkenes.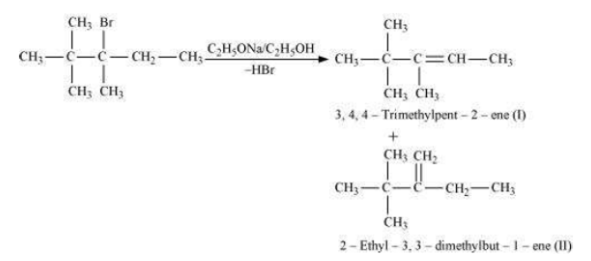
Saytzeff’s rule implies that in dehydrohalgenation reaction, the alkene having a greater number of alkyl groups attached to a doubly bonded carbon atoms is perferably produced. Hence, alkene (I) is the major product in this reaction.
 Long Answer Type
Long Answer Type