 Short Answer Type
Short Answer TypeExplain why the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
 Long Answer Type
Long Answer TypeGive the uses of Freon 12, DDT, carbon tetrachloride and iodoform.
Freon-12: The chlorofluorocarbon compounds of methane and ethane are collectively known as ferons.
Dichloro difluoro methane (Freon 12) is the most common freons. It is manufactured from tetra-chloromethane by the action of antimony trifluoride in the presence of antimony pentafluoride.
Uses:
(a) It is used as a refrigerant (cooling agent) in refrigerators and air conditioners.
(b) It is also used as a propellant in aerosols and foams to spray out deodorants, cleaners, hair sprays, shaving creams.
(c) It is also used as insecticides.
(ii) DDT (p, p’ - Dichlorodiphenyl trichloro ethane):
Preparation: It is manufactured by the condensation of chlorobenzene with trichloro acetaldehyde (chloral) in the presence of sulphuric acid.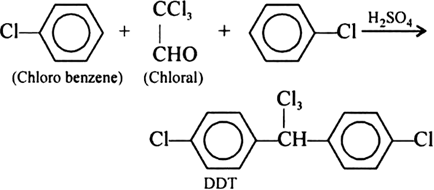
Uses:
(a) It is a powerful insecticide. It is highly stable and not easily decomposed.
(b) It is used for killing insects and mosquitoes.
(iii) Carbon tetrachloride (CCl4) : Preparation: It is prepared industrially by chlorination of methane and by the action of chlorine on carbon disulphide in the presence of aluminium chloride as catalyst.
Uses:
(a) It is used as a solvent for oils, fats and waxes.
(b) It is used as a fire extinguisher under the name pyrene.
(c) It is used as dry cleaning.
(d) It is used for the manufacture of freon.
(iv) Iodoform (CHI3):
Preparation: It is prepared by using ethanol or acetone with sodium hydroxide and iodine or sodium carbonate and iodine in water.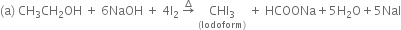

Uses:
(a) It is used as an antiseptic and this nature is due to iodine that it liberates. However, because of its very unpleasant smell, it has now been replaced by better antiseptics.
(b) It is used in the manufacture of pharmaceuticals.
 Short Answer Type
Short Answer Type