 Short Answer Type
Short Answer TypeChlorobenzene is extremely less reactive towards a nucleophilic substitution reaction. Give two reasons for the same?
1) Resonance effect: The electron pair on chlorine atom is in conjugation with the electrons of the benzene ring which results in the following resonance structures:
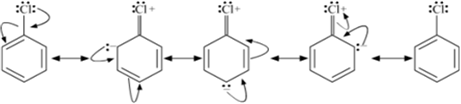
This results in delocalization of the electrons of C- Cl bond and a partial double bond character develop in the bond, which makes it difficult for the nucleophile to cleave the C- Cl bond.
2) The nucleophile suffers repulsion from the increased electron density on the benzene ring, as a result, the nucleophile is unable to make a close approach for the attack on the molecule.
Although chlorine is an electron withdrawing group, yet it is ortho-, Para-directing in electrophilic aromatic substitution reactions. Explain why it is so?
 Long Answer Type
Long Answer TypeAnswer the following:
(i) Haloalkanes easily dissolve in organic solvents, why?
(ii) What is known as a racemic mixture? Give an example.
(iii) Of the two Bromo derivatives, C6H5CH(CH3)Br and C6H5CH(C6H5)Br, which one is more reactive in Sn1 substitution reaction and why?
 Short Answer Type
Short Answer Type