 Short Answer Type
Short Answer TypeGive reasons for the following:
(i) Phenol is more acidic than ethanol.
(ii) The boiling point of ethanol is higher in comparison to methoxymethane.
(iii) (CH3)3C - O - CH3 on reaction with HI gives CH3OH and (CH3)3C -I as the main products and not (CH3)3C -OH and CH3I.
Give reasons for the following:
(i) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide.
(ii) (±) 2-Butanol is optically inactive.
(iii) C -X bond length in halo benzene is smaller than C -X bond length in CH3- X.
(i) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide because in the periodic table size increase if we move down. With an increase in size, basicity decrease, and the ability of the leaving group to leave increase. Iodine is good leaving the group thus it undergoes SN2 reaction faster than ethyl bromide.
(ii) Optically active compounds are those which rotates plane polarised light either left or right direction. In the case of (±) 2-Butanol is optically inactive because it is both dextrorotatory i.e. (+) and laevorotatory i.e. (-) and hence forms a racemic mixture in which the net rotation of plane-polarized light towards the right is cancelled by the left one and so it becomes optically inactive.
(iii) C—X bond length in halobenzene is lower than C—X bond length in CH3—X because in halo- benzene the C—X acquires partial double bond character due to resonance as shown below whereas in CH3—X there is no such resonance. As the bond length of the double bond is smaller than single bond hence C—X bond length in halo- benzene is smaller.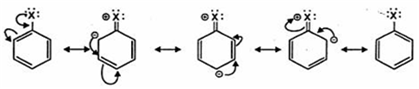
Answer the following question:
(i) What is meant by the chirality of a compound? Give an example.
(ii) Which one of the following compounds is more easily hydrolyzed by KOH and why?
CH3CHCICH2CH3 or CH3CH2CH2Cl
(iii) Which one undergoes S N 2 substitution reaction faster and why?![]()
Given reasons:
(i)C–Cl bond length in chlorobenzene is shorter than C–Cl bond length in CH3–Cl.
(ii)The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
(iii)SN1 reactions are accompanied by racemization in optically active alkyl halides.
Write the structure of an isomer of compound C4H9Br which is most reactive towards SN1 reaction.
