 Multiple Choice Questions
Multiple Choice QuestionsMatch List-I with List-II and select the correct answer from the given codes.
| List - I (Reaction) | List - II (Reagent/ Catalyst) |
| A. Cannizaro reaction | 1. SnCl2/ HCl |
| B. Stephen's reaction | 2. NaOH |
| C. Clemmensen reduction | 3. Zn/ Hg-conc. HCl |
| D. Rosenmund's reaction | 4. |
A - 1; B - 2; C - 3; D - 4
A - 2; B - 1; C - 3; D - 4
A - 4; B - 3; C - 2; D - 1
A - 1; B - 4; C - 2; D - 3
Haloalkane in the presence of alcoholic KOH undergoes
elimination
polymerisation
dimerisation
substitution
Grignard reagent is not prepared in aqueous medium but prepared in ether medium. Because
the reagent reacts with water
the reagent becomes inactive in water
it is insoluble in water
the reagent is highly reactive in water
Match List-I with List-II and select the correct answer using the codes given below.
| List I (IUPAC name of compound) | List II (common name of compound) |
| A. Propanone | 1. Dimethyl acetylene |
| B. Trichloromethane | 2. Methyl acetic acid |
| C. Propanoic acid | 3. Chloroform |
| D. But-2-yne | 4. Acetone |
A B C D
1 2 3 4
A B C D
4 3 2 1
A B C D
3 4 1 2
A B C D
1 3 2 4
Which of the following statements is incorrect regarding benzyl chloride?
It gives white precipitate with alcoholic AgNO3
It is an aromatic compound with substitution in the side chain
It undergoes nucleophilic substitution reaction
It is less reactive than vinyl chloride
D.
It is less reactive than vinyl chloride
Benzyl chloride is very reactive. It readily give, white precipitate with alcoholic AgNO3 at room temperature. It also readily undergo nucleophilic substitution. Its structure is as follows:
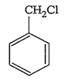
Vinyl chloride (CH2= CH.Cl), on the other hand, is less reactive than benzyl chloride due
to resonance.
![]()
The order of reactivities of methyl halides in the formation of Grignard reagent is
CH3I > CH3Br > CH3Cl
CH3Cl > CH3Br > CH3I
CH3Br > CH3Cl > CH3I
CH3Br > CH3I > CH3Cl
Which one of the following compounds gives trichloromethane on distilling with bleaching powder?
Methanal
Phenol
Ethanol
Methanol
Which of the following haloalkanes is most reactive?
1-chloropropane
1-bromopropane
2-chloropropane
2-bromopropane
