 Multiple Choice Questions
Multiple Choice QuestionsAn incorrect statement with respect to SN1 and SN2 mechanisms for alkyl halide is
A strong nucleophile in an aprotic solvent increases the rate or favours SN2 reaction.
Competing reaction for an SN2 reaction is rearrangement.
SN1 reactions can be catalysed by some Lewis acids.
A weak nucleophile and a protic solvent increases the rate or favours SN1 reaction.
The hydrolysis of optically active 2-bromobutane with aqueous NaOH result in the formation of
(+) butan -2-ol
(-) butan-2-ol
(±) butan-1-ol
(±) butan-2-ol
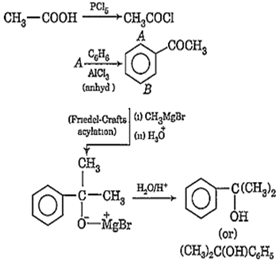
Predict the product 'C' in the following series of reactions:
CH3-COOH
![]()
CH3CH(OH)C6H5
CH3CH(OH)C2H5
(CH3)2C(OH)C6H5
D.
(CH3)2C(OH)C6H5
C is
In Grignard reagent the carbon-magnesium bond is:
electrovalent
covalent
dative
hydrogen bonding
In the reaction,
CH3CH2C ≡ CH Products
Products will be:
CH3COCH3
CH3COCH2OH
CH3COOH + HCOOH
CH3CHO + HCHO
The compound formed on heating chlorobenzene with chloral in the presence of concentrated sulphuric acid is
gammexane
DDT
freon
hexachloroethane
Ethyl alcohol is used as a preservative for chloroform because it :
prevent aerial oxidation of chloroform
prevents decomposition of chloroform
decomposes phosgene to CO and Cl2
removes phosgene by converting it to ethyl carbonate
