Multiple Choice Questions
Multiple Choice QuestionsAssertion : Alkyl iodide can be prepared by treating alkyl chloride/bromide with NaI in acetone.
Reason : NaCl/NaBr are soluble in acetone while NaI is not.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
SN2 mechanism is involed in the following substitution :
CH3-CH2-Cl + OH-
(CH3)2-C(Cl)-CH3-OH-
(CH3)2-C-Cl + OH-
CH3-CH2-(CH3)C(Cl)-CH3 + OH-
Arrange the hydra - acids of halogens in increasing order of acidity .
HF < HCl < HBr < HI
HI < HBr < HCl < HF
HF < HBr < HI <HCl
HF < HI < HBr < HCl
IUPAC name of
CH3CH2C(Br)=CH-Cl is :
2 - bromo - l - chloro butene - 1
1 - chloro - 2 - bromo - butene
3 - chloro - 2 - bromo butene - 2
none of these
Which of the following sequence of reactions (reagents) can be used for the conversion of C6H5CH2CH3 into C6H5CH=CH2?
SOCl2 ; H2O
SO2Cl2; alc. KOH
Cl/h; H2O
SOCl2 ; alc. KOH.
Which ofthe following compounds has the highest boiling point?
CH3CH2CH2Cl
CH3CH2CH2CH2Cl
CH3CH(CH3)CH2Cl
(CH3)3CCl
The correct increasing order of the reactivity of halides for SN 1 reaction is
CH3- CH2- X<(CH3)2CH= X <CH2= CH-CH2- X< PhCH2- X
(CH3)2CH-X <CH3-CH2- X < CH2= CH - CH2-X < PhCH2-X
PhCH2- X<(CH3)2CH=X<CH3-CH2-X<CH2= CH- CH2- X
CH2= CH - CH2- X < Ph- CH2- X <(CH3)2CH-X <CH3-CH2- X
The major product formed in the following reaction is

(CH3)2CH-CH2OCH3
CH3-CH(OCH3)-CH2CH3
CH3-C(CH3)=CH2
(CH3)2-C-OCH3- CH3
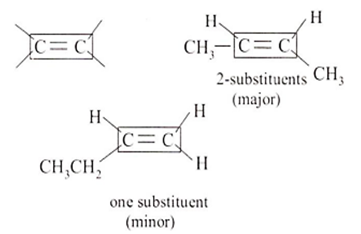
The major product obtained on treatment of CH3CH2CH(F)CH3 with CH3O/CH3OH is
CH3CH2CH(OCH3)CH3
CH3CH = CHCH3
CH3CH2CH = CH2
CH3CH2CH2CH2OCH3
B.
CH3CH = CHCH3
According to Saytzeff's rule, the major product will be the one that contains more number of substituents around the double bond. Therefore, the correct answer is CH3CH = CHCH3.