 Long Answer Type
Long Answer TypeWrite structures of different chain isomers of alkanes corresponding to the molecular formula C6H14. Also, write their IUPAC names.
Write structures of different isomeric alkyl groups corresponding to the molecular formula C5H11. Write IUPAC names of alcohols obtained by attachment of -OH groups at different carbons of the chain.
Write IUPAC names of the following compounds:
(i) (CH3)3 C CH2C(CH3)3
(ii) (CH3)2 C(C2H5)2
(iii) tetra-tert-butyl methane
 Short Answer Type
Short Answer TypeWrite structural formulas of the following compounds:
(i) 3, 4, 4, 5-Tetramethylheptane.
(ii) 2, 5-Dimethylhexane.
Write structures for each of the following compounds. Why are the given names incorrect? Write correct IUPAC names:
(i) 2-Ethylpentane
(ii) 5-Ethyl-3-methylheptane
 Long Answer Type
Long Answer TypeHow are alkanes formed from:
(i) unsaturated hydrocarbons
(ii) sodium salt of fatty acids?
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer bytaking one example.
Give a brief account of Kolbe's electrolysis.
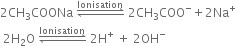
On passing electricity, the ions will move towards the respective electrodes.
At anode: The electron releasing tendency of CH3COO- ions is more and these are discharged in preference to OH- ions.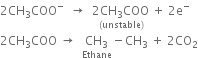
At cathode: The electron accepting tendency of H+ ions is more and these are discharged in preference to Na+ ions which remain in solution.

Limitations:
(i) Only alkanes with an even number of carbon atoms can be formed.
(ii) Methane cannot be prepared by this method.
 Short Answer Type
Short Answer Type