 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeOut of staggered and eclipsed conformations of ethane, which is more stable and why ?
Staggered conformation of ethane is more stable than the eclipsed conformation.
Reason. When the staggered conformation is rotated through an angle of 60°, it changes to eclipsed conformation and the eclipsed conformation when further rotated through 60° gives back the staggered conformation.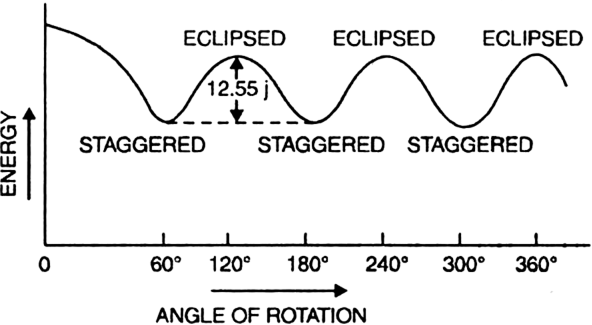
These two conformations differ in their energy contents and also in their relative stabilities. The staggered conformation with minimum repulsions between C-H bonding electrons and hydrogens of one methyl group and those of other has lesser energy than the eclipsed conformation where the force of repulsion between C-H bonding electrons and hydrogen of one methyl group and those of other is maximum.
The energy difference between staggered and eclipsed conformation of ethane is 12-55 kJ/mole. Hence staggered conformation is more stable than the eclipsed conformation.
 Short Answer Type
Short Answer Type