 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeGive IUPAC names of the following compounds:
(a) CH3CH = (CH3)2
(b) CH2 = CH – C ≡ C – CH3
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWrite structure and IUPAC names of different structural isomers of alkenes corresponding to C5H10.
What is meant by hindered (or restricted) rotation around carbon-carbon double bond? What type of isomerism does it lead to?
Hindered rotation about double bond: The carbon-carbon double bond in ethylene and other compounds consists of a σ bond and a 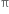 bond. The
bond. The 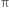 bond involves the overlapping of unhybridized p-orbitals of two carbon atoms above and below the plane of the atoms. Because of this type of overlapping, rotation around carbon-carbon double bond is strongly hindered and can do so only if the
bond involves the overlapping of unhybridized p-orbitals of two carbon atoms above and below the plane of the atoms. Because of this type of overlapping, rotation around carbon-carbon double bond is strongly hindered and can do so only if the 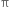 bond breaks. The breaking of the
bond breaks. The breaking of the 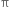 bond requires about 284 kJ of energy per mole which is not available to the molecule at room temperature. Hence, the atoms or groups attached to each carbon atom can not rotate about it. The rotation about the double bond is, therefore, restricted or hindered.
bond requires about 284 kJ of energy per mole which is not available to the molecule at room temperature. Hence, the atoms or groups attached to each carbon atom can not rotate about it. The rotation about the double bond is, therefore, restricted or hindered.
Isomerism due to hindered rotation: Due to hindered rotation around carbon-carbon double bond, the relative positions of groups attached to the double bonded carbon atoms get fixed. As a result, several alkenes can exhibit geometrical isomerism.
 Short Answer Type
Short Answer TypeWhat are the necessary and sufficient conditions for a compound to exhibit geometrical isomerism ?
Name the various structural isomers possible in C4H8. Which one will exhibit geometrical isomerism?
