 Short Answer Type
Short Answer TypeWhat happens when:
(i) Ethyl alcohol is heated in the presence of Al2O3 at 493 K?
(ii) Ethylene dibromide is heated with zinc dust?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe 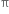 -bond is weaker than carbon-carbon σ bond. Explain.
-bond is weaker than carbon-carbon σ bond. Explain.
The >C = C< bond is made of σ bond and  bond. The
bond. The  bond is weaker than σ bond because electron in the
bond is weaker than σ bond because electron in the 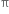 bond are more diffused in space i.e. orbitals participating in the
bond are more diffused in space i.e. orbitals participating in the 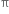 bond formation overlap sidewise only to small extent. On the other hand, orbitals participating in the σ bond formation overlap axially to a greater extent.
bond formation overlap sidewise only to small extent. On the other hand, orbitals participating in the σ bond formation overlap axially to a greater extent.
The greater the extent of overlapping, the higher the probability of finding the valence electrons in between the nuclei and hence the bond will be stronger and shorter. Hence overlap in 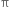 bond is less effective as compared to σ bond.
bond is less effective as compared to σ bond.
 Long Answer Type
Long Answer TypeDiscuss the mechanism of addition hydrogen acids to symmetrical alkenes. Justify the order of reactivity of halogen acids HI > HBr > HCl.
